Boiler water treatment philosophy and overview
Boiler basics and types of boilers | Common issues found on Boiler feed water | Boiler water treatment fundamentals | Water Treatment Philosophy and Overview |Boiler Water Treatments and its purpose
Boiler water treatment philosophy and overview
1 .TYPES OF WATER
General
- Water could generally be described as the most important of all chemical substances. Its chemical designation is H2O; the water molecule is composed of 2 Hydrogen atoms and 1 Oxygen atom.
Natural water
- Raw water is the description of the water to which we have daily access. We can obtain our water from:
1. The ocean
2. Surface sources (e.g. from lakes)
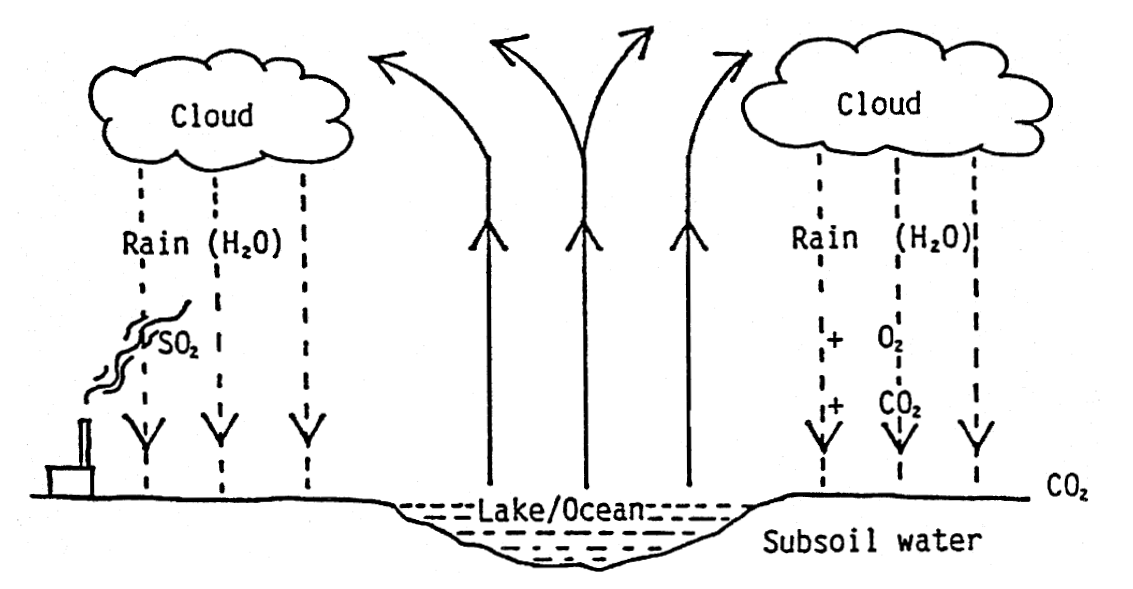
3. Underground sources - The water will vary in composition. The natural water cycle may be as below:
- While it is evaporating from the surface of a lake or the ocean into the atmosphere, we can designate the water vapour H2O. In the atmosphere, clouds will form, and during suitable humidity and temperature, the clouds will deposit water (rain). While the rain is falling towards the earth, it absorbs gases which are in the air, e.g. CO2 (Carbon Dioxide), SO2 (Sulphur Dioxide) and O2 (Oxygen).
- When the water hits the earth, it absorbs additional Carbon Dioxide (from biological degradation). The rainwater which is now slightly acid will dissolve various minerals from the soil.
2. Basic Chemistry
The chemistry of water
For more information on property of fresh water please read [1]
- It is necessary to examine some of the basic theories in order to understand the various problems associated with water treatment.
- While rain is falling through the air, it absorbs gaseous contaminants, e.g. O2 (Oxygen), which solubility in pure water depends on temperature.
- At 20 °C, 9 mg/l O2 may dissolve, and at 50 °C approx. 5.5 mg O2/l, and at 90 °C approx. 1.5 mg O2/l, and at 100 °C approx. 0.0. mg O2/l, so, the higher the temperature, the less O2 can dissolve in water.
- CO2 (Carbon Dioxide) dissolves in water as follows:
CO2 + H2O > H2CO3
H2CO3 is a very weak acid. In contact with CaCO3 (ordinary lime), it is reactive and the lime dissolves as follows: - CaCO3 + H2CO3 > Ca++ + 2HCO3 –
- Ca(HCO3)2 is called Calcium Bicarbonate.
- SO2 (Sulphur Dioxide) is an air pollutant which stems from flue gases, so there is usually a high atmospheric content of this gas around industrial areas.
- 2SO2 + O2 + 2H2O > 2H2SO4
H2SO4 is called Sulphuric Acid, and this acid also dissolves lime (CaCO3)
as follows:
CaCO3 + H2SO4 > CaSO4 + H2O + CO2. CaSO4 is called Calcium Sulphate (gypsum). - In other words, the gases dissolved in the water will increase the leaching of the subsoil’s minerals, so that we may have solutions in water due to:
| TOTAL HARDNESS | |
| Temporary hardness | Permanent hardness |
| Calcium Bicarbonate
Bicarbonate Calcium Sulphate Magnesium Bicarbonate Mg (HCO3)2 |
Calcium Sulphate
CaSO4 Magnesium Chloride MgCl2 |
- TEMPORARY HARDNESS (Alkaline Hardness) is due to bicarbonates of Calcium and Magnesium which are Alkaline in nature. They are “temporary” because when heated they rapidly break down to form Carbon Dioxide and the corresponding carbonates which deposit as scale.
- PERMANENT HARDNESS (Non-Alkaline Hardness) is due mainly to Sulphates and Chlorides of Calcium and Magnesium which are acid in nature. They are “permanent” and do not break down, but under certain conditions deposit to form scale of varying hardnesses.